Projects in detail
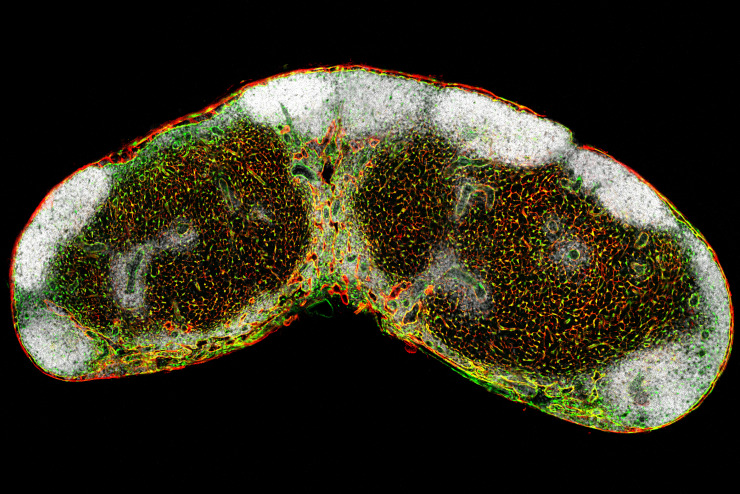
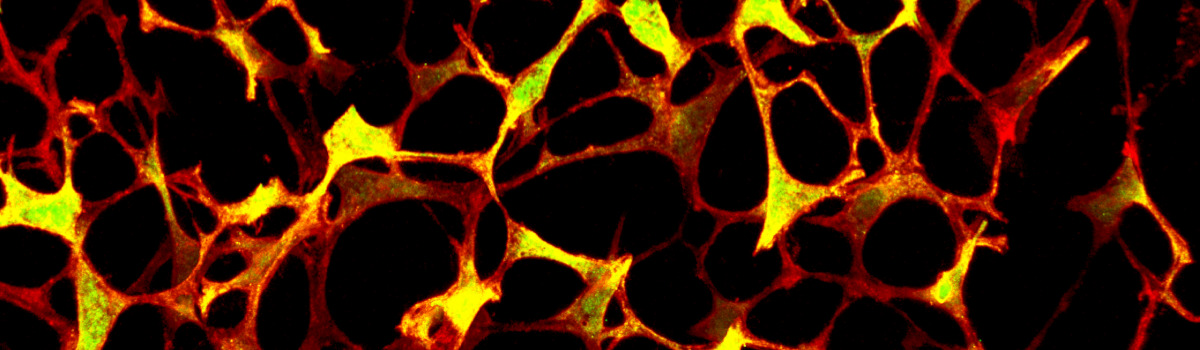
Lymph node FRC network
Defining the origin of lymph node and Peyer's patch fibroblastic reticular cells at single cell resolution
-
Secondary lymphoid organs such as lymph nodes (LNs) and Peyer’s patches (PPs) sample antigens from the body’s inner and outer surfaces and mediate optimal interaction of immune cells. These functions rely on the presence of specialized microenvironments that are built and maintained by fibroblastic reticular cells (FRCs). In addition to their scaffold-building function, FRCs impact on inducing and shaping innate and adaptive immune responses. However, we do not know the embryonic origin of these important cells and the molecular mechanisms underlying FRC differentiation in LNs or PPs. Our laboratory has generated the appropriate lineage-tracing and fate-mapping approach to address these questions. Accordingly, the work program of this project has been designed on the basis of two main hypotheses: (i) LN and PP FRCs originate from an organ-specific pluripotent embryonic precursor cell, and (ii) committed embryonic LN FRC progenitors descend from fat pad fibroblasts.
Only in recent years, the importance of secondary lymphoid organ stromal cells for induction and regulation of immune responsiveness has been appreciated. The field is now rapidly moving forward exploring functions and exploiting the potential of these cells as therapeutic targets in autoimmune diseases, cancer and infection. We anticipate that defining the origin of fibroblastic stromal cells and elaborating the mechanisms that govern subset differentiation in LNs and PPs will provide critical knowledge to further elaborate diagnostic and therapeutic avenues for various diseases.
Funded through Swiss National Science Foundation (SNF) Project Number 166500
Ontogeny and functional characterization of splenic fibroblastic reticular cells (FRCs) and their mesenchymal precursors during homeostasis, immune-activation and infection
-
Secondary lymphatic organs (SLOs) such as the spleen provide specialized microenvironmental niches for the development and control of immune responses. Particular mesenchymal stromal cells known as fibroblastic reticular cells (FRCs) generate distinct compartments that permit, for example, highly efficient interaction of T cells with dendritic cells. Importantly, FRCs are more than simple scaffold-building cells; these cells actively participate in inducing and shaping innate and adaptive immune responses. Our own preliminary data demonstrate that the structure and function of the splenic white pulp exclusively depends on lymphotoxin-beta-receptor (LTbR) signaling received by CCL19-positive FRCs during adult life and/or their putative progenitors, i.e. mesenchymal lymphoid tissue organizer cells (mLTOs).
In continuation of our existing collaborative activities, we have developed a novel inducible lineage-tracing model that permits genetic tagging of splenic FRCs and their progenitors in vivo and will thus facilitate the elucidation of their differentiation pathways throughout spleen development. In addition, this model system provides means for the identification and characterization of molecular mechanisms involved in the maintenance of splenic structure and immune-competence during adult life. Thus, in combination with our already established experimental model of cell type-specific loss of LTbR function, we will be able to resolve the temporal requirement of LTbR signaling for differentiation and function of adult splenic FRCs and their putative progenitors in the embryo.
Finally, using an experimental model that facilitates cell type-specific re-activation of LTbR expression in a timely controlled manner, we will test the hypothesis whether FRC-restricted LTbR expression within a specific time window is sufficient for proper white pulp formation and function during embryonic life. Furthermore, we will elucidate the requirements for a timely controlled LTbR activation during adult life in order to maintain compartmentalization and function of the splenic white pulp.
Our proposed objectives are based on our individual expertise and present the continuation of our successful collaborative activities. More specifically, they are designed to molecularly characterize splenic FRCs and to dissect lineage-relationship of splenic FRCs with other splenic stromal cells, e.g. their differentiation from mLTO precursors during embryonic development. Moreover, we will be able to identify and functionally characterize the cellular and molecular mechanisms through which LTbR–expressing FRCs are involved in the maintenance of lymphoid organ integrity and immune-competence in the adult spleen, i.e. their ecological role in the splenic micro-environment during immune homeostasis, activation and infection.Funded through Swiss National Science Foundation (SNF): 159188
Defining the identity and differentiation pathways of the immune-stimulating fibroblastic tumor stroma
-
The tumor stroma has become an important area of research in recent years following the notion that stromal cell targeting therapies may provide a common therapeutic target for many tumor types. Although numerous studies have addressed the ways by which neoplastic cells alter their environment the origin, function and identity of fibroblastic cell lineages in the tumor microenvironment (TME) have remained largely unknown. These lapses in our basic knowledge of fibroblast differentiation in the tumor microenvironment make it difficult to define tumor-promoting versus tumor-suppressive fibroblasts, identify mechanisms of immune-stimulating fibroblast differentiation and their relation with tumor-associated tertiary lymphoid structures (TLS), and extrapolate large-scale bioinformatics analyses from cells in the bulk tumor to delineate cell- and lineage-specific differentiation pathways.
The overarching goal of the project is to define the differentiation and function of immune-stimulating fibroblasts within the lung following tumor-induced activation. This project is built on our working hypothesis that the lung stroma is composed of multiple fibroblast lineages, which exhibit immune-stimulating potential in the TME. Differentiation of immune-stimulating fibroblasts fosters the formation of TLS, which are niches that promote antitumor immunity and as such have an overall tumor-suppressive effect. To experimentally evaluate our model, we will combine the expertise and state-of-the-art models and methods from experimental and quantitative biology with a powerful computational approach to gain a comprehensive view of the fibroblastic lung tumor stroma.
The use of genetic models that label immune-stimulating fibroblasts will permit us to visualize and manipulate defined fibroblast lineages and to isolate these cells for further characterization. Using lung tumor models that exhibit peritumoral TLS, we will examine the distribution of immune-stimulating fibroblasts within the healthy versus lung tumor stroma and associated TLS. Furthermore, cell-specific ablation will reveal the functional impact of immune-stimulating fibroblasts on TLS formation and antitumor immunity. Having pinpointed relevant, tumor-associated fibroblast subsets, we will utilize new computational tools to create a data-based model of immune-stimulating fibroblast maturation in mice, which will serve as a platform to extrapolate related human fibroblast lineages. To this end, we will perform RNA-seq and mass cytometry on the single-cell level to comprehensively define phenotypes and functional states of fibroblasts in human lung tumors. The molecular map of human lung tumor fibroblast differentiation will be validated by single-cell transcriptomic and proteomic analysis of fibroblasts in tumor-free lung regions of the patients suffering from adenocarcinoma or squamous cell carcinomas. Overall, we expect that these studies will reveal putative therapeutic targets to bias the differentiation of immune-stimulating fibroblasts within the TME.
Funded through Swiss National Science Foundation (SNF): 177208
Dissecting the association of the human palatine tonsil microbiome and HPV-driven oropharyngeal squamous cell carcinoma
-
Oropharyngeal squamous cell carcinoma (OPSCC) can be caused by viral transformation of epithelial cells in the oropharynx such as high risk-human papilloma virus (HR-HPV). Importantly, the incidence of OPSCC has been increasing during the last decades although tumors with biologically active infection by HR-HPV exhibit a distinct biology and improved treatment response leading to better patient outcome. Recent studies have suggested that the containment of HR-HPV can be influenced by the microbiome present at mucosal surfaces and by microbial agents that persist within immune cells and thereby alter immune responses against the pathogen. Hence, we hypothesize that the microbiome of human palatine tonsils contributes to the establishment and persistence of HPV infection leading to malignant transformation and development of OPSCC. In particular, we assume that the microbial composition in left and right tonsils from the healthy individual is identical or at least very similar. Moreover, we expect to detect differences between the diseased and healthy tonsils of patients affected by OPSCC.
We plan to characterize the microbial composition of tonsillar surfaces and crypts in healthy individuals suffering from sleep apnae using next generation sequencing of 16S V5-V6 rDNA amplicons. We expect that this initial part of the study will fill a critical gap in the knowledge on the microbiome present in different compartments in tonsils of healthy adults. Next, we plan to profile the tonsillar microbiome of patients suffering from HPV-associated OPSCC and OPSCC that is not linked to HPV infection. We expect that evaluation of next generation sequencing and clinical data will provide novel knowledge on microbiome-HPV interaction and will found the basis for further prospective clinical studies. Finally, we plan to assess whether a distinct intracellular microbiome exists in immune cells within the tonsillar lymphoid tissue using multi-parameter flow cytometric analysis and cell sorting. We anticipate that these analyses will reveal whether and to what extent bacterial communities are associated with immune cells in human tonsils.
Overall, the outlined project will provide hitherto unknown information on the microbiome of different compartments in human tonsils, support design of future prospective clinical studies to clarify the role of the microbiome in HVP-driven OPSCC and direct future analyses of immune cell-commensal bacteria interaction in tonsillar lymphoid tissue.Funded by the San Salvatore Foundation
Constructing accessory lymph nodes in situ for control of mammary carcinoma
-
Lymph nodes (LNs) are situated at junctures of the blood vascular and the lymphatic system where antigens drain from peripheral tissues via afferent lymphatics. The decision whether and how immune responses against tumors are initiated and maintained is made in local tumor-draining LNs. During the development of malignant breast cancer, new LNs emerge within the glandular tissues that are normally devoid of LNs. However, we do not understand the mechanism of development of these LNs and their role in antitumor immunity. Thus, the novel hypothesis underlying the planned research is that de novo LNs appear in the vicinity of breast cancer as an accessory “base camp” for the initiation and maintenance of antitumor immunity. Here, we combine and leverage knowledge and experimental tools in immunology, vascular biology, cancer biology, and bioengineering to address the hypothesis in three specific aims:
1) To dissect the molecular pathways of tumor-induced development of accessory (a)LNs with a particular emphasis on lymphovasculokines.
2) To determined to which extent aLNs support antitumor immunity.
3) To stimulate formation of aLNs in mammary tissues to foster antitumor immunity.The project is conceptually innovative because a deeper understanding of the interplay between aLNs and tumors may not only lead to a novel approach to cancer treatment, but could also help to establish a novel prevention paradigm in cancer medicine. The aims of the project will be addressed in a cooperative manner and activities in each group will contribute to address the global aims of the project. Gain of knowledge on the basic molecular principles involved in aLN formation (major aim 1) will absorb the major part of the activities. Determination of the impact of aLNs on antitumor immunity (major aim 2) will be assessed in cooperation between the three groups. Finally, formation of aLNs in tumor-draining areas in vivo (major aim 3) will be assessed by using highly innovative approaches including implantation of LN-like stromal cell spheroids and optogenetic induction of molecules that foster activation of the aLN anlage. In sum, the combination of the special expertise of the applicants in LN development, lymphovasculokine biology and optogenetic technology is crucial for addressing the ambitious project aims and therefore secures the basis for successful execution of the research plan.
Funded through Human Frontier Science Program (HFSP)